Popular on Rezul
- Tatanka Run Announces Phase-2 Development Following Completion of Phase-1 Spec Home
- Inside the Fight for Affordable Housing: Avery Headley Joins Terran Lamp for a Candid Bronx Leadership Conversation
- Controversial Vegan Turns Rapper Launches First Song, "Psychopathic Tendencies."
- Data Over Drama: Market Trends 2026 to discuss what's next for Florida's real estate market
- Zacuto Group Brokers Sale of 1936 Mateo Street in Downtown Los Angeles
- Beucher Insurance Agency Responds to Historic Rain Event in North Lake County Florida
- Laurie McLennan and The McLennan Team Announces Strong Finish to 2025 With Notable Luxury Sales in La Quinta and Palm Desert
- UK Financial Ltd Announces A Special Board Meeting Today At 4PM: Orders MCAT Lock on CATEX, Adopts ERC-3643 Standard, & Cancels $0.20 MCOIN for $1
- From Cheer to Courtroom: The Hidden Legal Risks in Your Holiday Eggnog
- T-TECH Partners with Japan USA Precision Tools for 2026 US Market Development of the New T-TECH 5-Axis QUICK MILL™
Similar on Rezul
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
- Psychiatric Drug Damage Ignored for Decades; CCHR Demands Federal Action
- Why Millions Are Losing Sexual Sensation, And Why It's Not Age, Hormones, or Desire
- Russellville Huntington Learning Center Expands Access to Literacy Support; Approved Provider Under Arkansas Department of Education
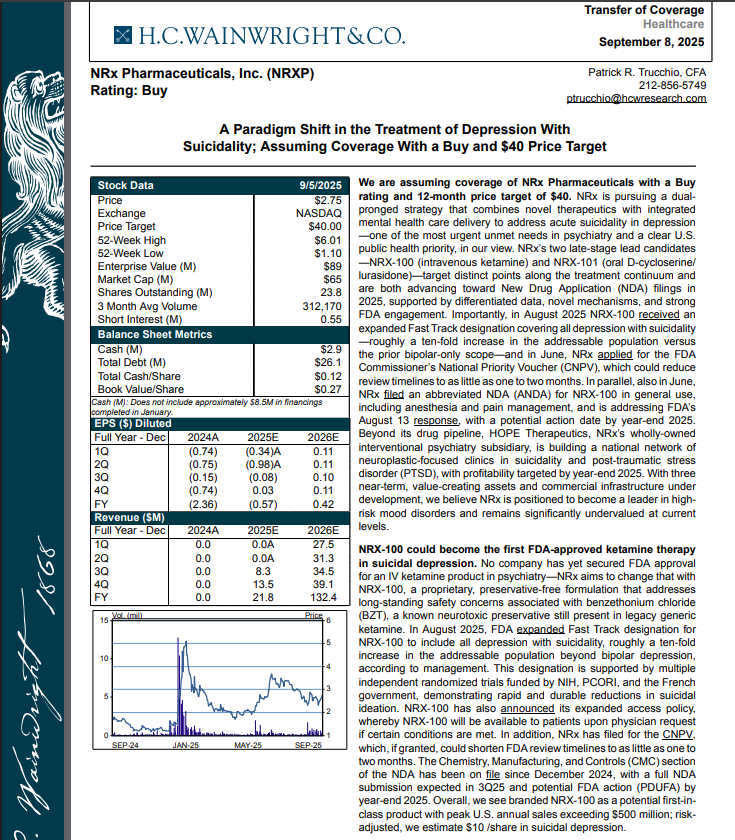
$750 Million Market Projected to Reach $3.35 Billion; Huge Opportunity for Superior Preservative-Free Ketamine Drug Treating Suicidal Depression $NRXP
Rezul News/10716179
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Refiles Abbreviated New Drug Application; $40 Price Target in New H. C. Wainright Research Report
MIAMI - Rezul -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Re-Filing of Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
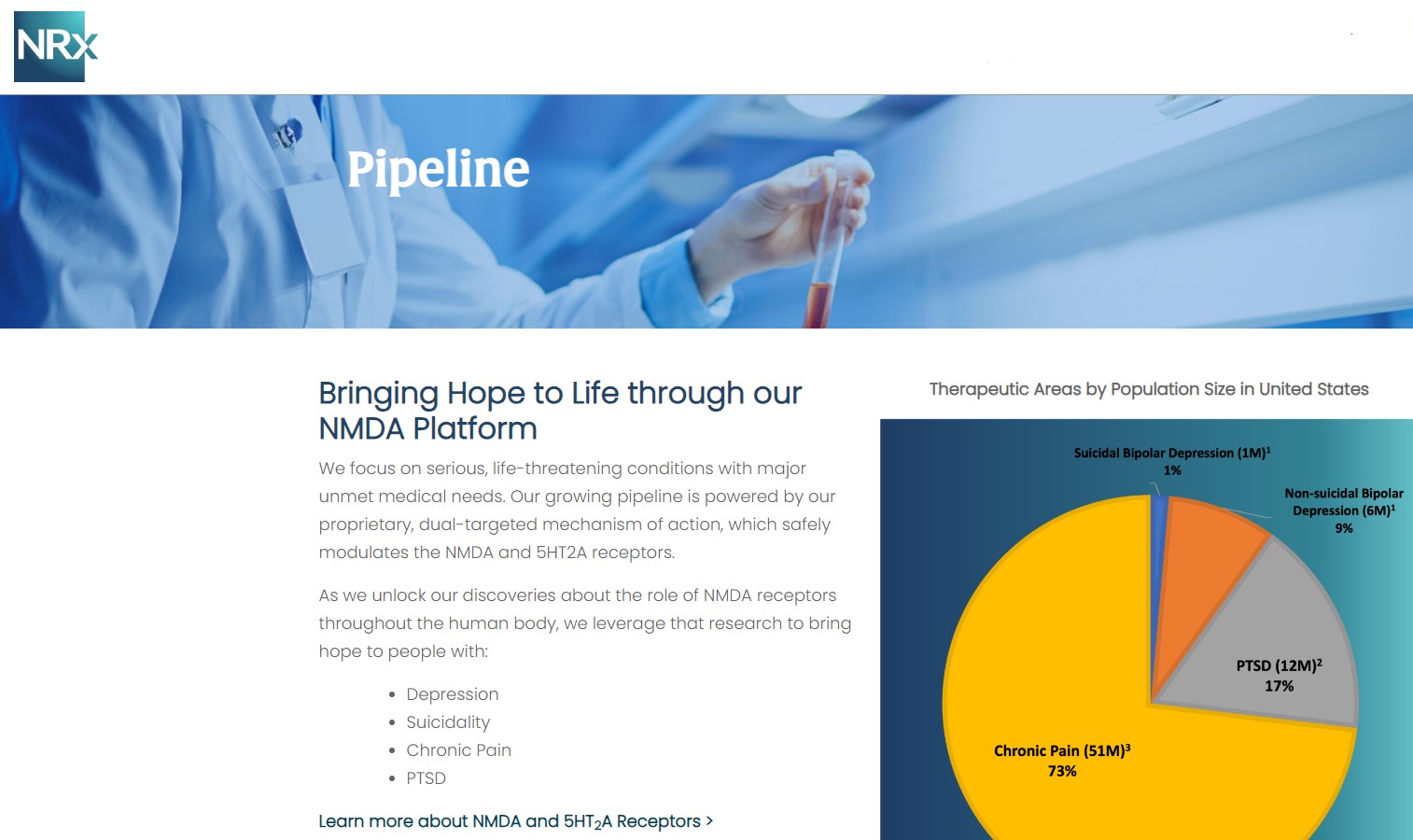
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on Rezul News
NRXP Re-Files Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine
On September 29th NRXP announced the re-filing of its Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration (FDA) for KETAFREE™, its preservative-free IV ketamine formulation, for use in all existing approved indications. The filing follows FDA grant of approval of its Suitability Petition for the NRXP proposed strength of preservative-free ketamine.
The current annual ketamine market is estimated at $750 million, with global demand for ketamine projected to grow to $3.35 billion by 2034. This does not include the widespread use of compounded ketamine by clinics unable to obtain manufactured drug. NRx aims to capture a significant share of the current market. According to a 2021 survey, an estimated 5.1 million Americans had received ketamine for medical uses in their lifetime3, a number that continues to grow with increased clinical focus on this important medication. Ketamine currently faces a severe drug shortage according to the American Society of Hospital Pharmacists. Accordingly, NRXP is seeking priority review from FDA.
NRXP previously filed a citizen's petition with the FDA to remove benzethonium chloride (BZT), a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. The FDA has previously disallowed the use of BZT in hand cleansers and topical antiseptics. A related preservative, benzalkonium chloride has demonstrated corneal and conjunctival toxicity in artificial tears and glaucoma medications, leading to use of preservative-free alternatives. NRXP has filed expert testimony from accredited toxicologists regarding the toxicity of BZT, which is not generally recognized as safe (GRAS) by the FDA. Removal of potentially harmful preservatives from foods is a stated priority in the MAHA report and HHS leadership has additionally targeted preservatives in vaccines. BZT was originally added to ketamine when it was first formulated in the 1970s to maintain stability and sterility using the container closure systems then available. NRx has demonstrated long term stability and sterility with a patented preservative-free formulation using modern manufacturing methods.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
More on Rezul News
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Re-Filing of Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on Rezul News
- Comanche Christmas Parade Wraps the Town in Holiday Cheer
- Guests Can Save 25 Percent Off Last Minute Bookings at KeysCaribbean's Village at Hawks Cay Villas
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- American Net Lease Facilitates Sale of Dollar General in Conroe, Texas
NRXP Re-Files Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine
On September 29th NRXP announced the re-filing of its Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration (FDA) for KETAFREE™, its preservative-free IV ketamine formulation, for use in all existing approved indications. The filing follows FDA grant of approval of its Suitability Petition for the NRXP proposed strength of preservative-free ketamine.
The current annual ketamine market is estimated at $750 million, with global demand for ketamine projected to grow to $3.35 billion by 2034. This does not include the widespread use of compounded ketamine by clinics unable to obtain manufactured drug. NRx aims to capture a significant share of the current market. According to a 2021 survey, an estimated 5.1 million Americans had received ketamine for medical uses in their lifetime3, a number that continues to grow with increased clinical focus on this important medication. Ketamine currently faces a severe drug shortage according to the American Society of Hospital Pharmacists. Accordingly, NRXP is seeking priority review from FDA.
NRXP previously filed a citizen's petition with the FDA to remove benzethonium chloride (BZT), a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. The FDA has previously disallowed the use of BZT in hand cleansers and topical antiseptics. A related preservative, benzalkonium chloride has demonstrated corneal and conjunctival toxicity in artificial tears and glaucoma medications, leading to use of preservative-free alternatives. NRXP has filed expert testimony from accredited toxicologists regarding the toxicity of BZT, which is not generally recognized as safe (GRAS) by the FDA. Removal of potentially harmful preservatives from foods is a stated priority in the MAHA report and HHS leadership has additionally targeted preservatives in vaccines. BZT was originally added to ketamine when it was first formulated in the 1970s to maintain stability and sterility using the container closure systems then available. NRx has demonstrated long term stability and sterility with a patented preservative-free formulation using modern manufacturing methods.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
More on Rezul News
- American Net Lease Facilitates Acquisition of Bojangles in Hartsville, SC
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- A Symphony of Support: Trang & David Hooser Champion Arts Education for Autistic Youth at NSA's Annual Gala
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Comanche Methodist Church Serves 500 Meals, Strengthening Community Connections
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Financial
0 Comments
Latest on Rezul News
- RealEstateRelated.com Expands AI Platform Following Pre-Seed Equity Round
- New Active Adult Ranch Homes by O'Dwyer Now Selling at Highly Anticipated Lake Society on Lake
- O'Dwyer Homes Introduces Bridgeview, New Semi-Custom Homes near Downtown Canton
- Pinealage: the app that turns strangers into meditation companions — in crowdfunding phase
- OKC Roofer Releases "Ultimate End-of-Year Roof Checklist" to Help Homeowners Prepare for Winter
- "Micro-Studio": Why San Diegans are Swapping Crowded Gyms for Private, One-on-One Training at Sweat Society
- Beycome Closes $2.5M Seed Round Led by InsurTech Fund
- Tatanka Run Announces Phase-2 Development Following Completion of Phase-1 Spec Home
- Tru by Hilton Columbia South Opens to Guests
- Christy Sports donates $56K in new gear to SOS Outreach to help kids hit the slopes
- PulteGroup Northeast Florida's 4th Annual Building Hope Golf Tournament raises record $224,331
- Newest David Weekley Homes Community Now Open In Georgia's Forsyth County
- "BigPirate" Sets Sail: A New Narrative-Driven Social Casino Adventure
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- Women's Everyday Safety Is Changing - The Blue Luna Shows How
- Microgaming Unveils Red Papaya: A New Studio Delivering Cutting-Edge, Feature-Rich Slots
- Why Buying a Home at Christmas will be Your Best Christmas Ever
- Adam's Plumbing & Heating Unveils the Ultimate Lakewood Plumbing Repair & Installation Resource for Homeowners and Businesses
- Hendricks Property Management #1 Property Manager in San Antonio & #27 Nationwide | Proudly Local
- 5-Star Duncan Injury Group Expands Personal Injury Representation to Arizona