Popular on Rezul
- Final Countdown: The OpenSSL Conference 2025 Begins in One Week - 102
- David White DDS Advances Implant Dentistry with New Technology Acquisition
- Titan Pressure Washing Showcases Professional Concrete Driveway & Walkway Cleaning in New South Florida Video
- Sine Nomine Associates: OpenAFS Google Summer of Code projects have been completed
- New Frontier Aerospace Appoints Industry Veteran Rich Pournelle as Director of Business Development
- Lineus Medical Obtains CE Mark for Flagship Product SafeBreak Vascular
- AI's Urgent Energy Requirements Won't Be Solved By Trillions Of Dollars. Phinge's Patented App-Less Netverse Platform & Hardware Will Reduce This Need
- Buyers Advocate Perth Marks Two Years of Redefining the Property Buying Experience
- DAECO Painting Sets the Gold Standard for High-End Interior Painting Services in Denver, CO
- High-End Home Renovation Tips for Denver | Truss Interiors
Similar on Rezul
- PatientNow Acquires Recura, the AI Growth Engine Powering Practice Growth
- Boston Industrial Solutions Unveils New and Improved Natron® UV Screen Printing Ink
- Genuine Smiles Unveils New User-Friendly Website
- Lift Solutions Holdings Announces Exclusive Distributorship for Advanced Camera and Sensor Products from Automate Matrix
- The Citizens Commission on Human Rights of Florida Celebrates Volunteers and Community Partners at the 9th Annual Humanitarian Awards Banquet
- Mature Athlete - Want Elite, Web-Based Nutrition and Training Coaching?
- Launch of Professional Private Autopsy Services to Support Families, Professionals, and Researchers
- TRUE Palliative Care Launches as California Strengthens Commitment to Compassionate Care Under SB 403
- Mysterious Interstellar Object 3I/ATLAS Appears to Pause Near Mars, Exhibiting Periodic Light Pulses
- $73.6 Million in Order Backlog Poised for Explosive Growth in 2026; Streamlined Share Structure: Cycurion, Inc. (N A S D A Q: CYCU) $CYCU
Lineus Medical Obtains CE Mark for Flagship Product SafeBreak Vascular
Rezul News/10716312
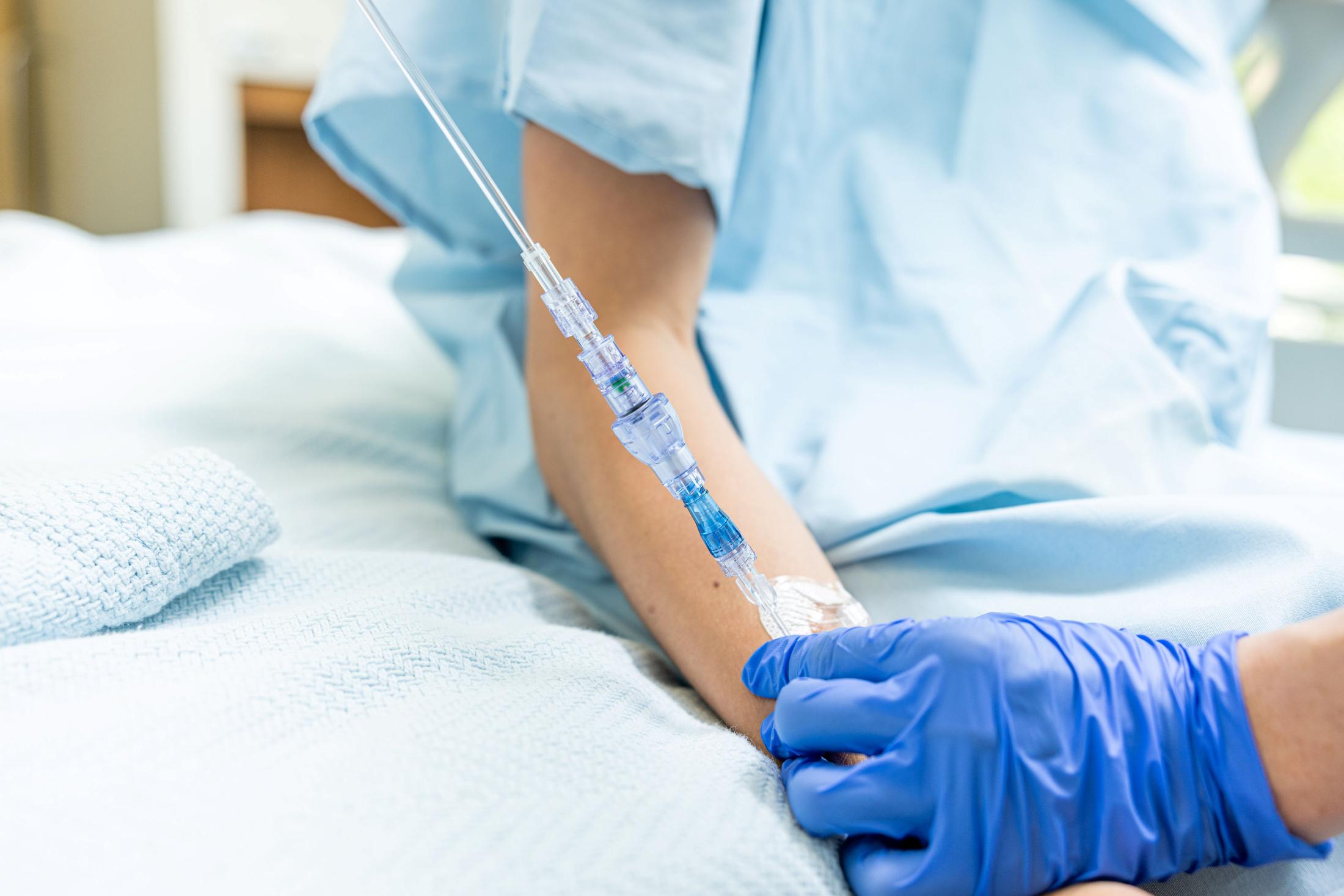
FAYETTEVILLE, Ark. - Rezul -- Lineus Medical has obtained CE Mark certification for its flagship product, SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 With this certification, SafeBreak Vascular is now cleared for use across the European Union. This approval expands SafeBreak Vascular's clearance from 5 countries to 32, spanning across more than three continents. Lineus Medical is actively partnering with distributors across Europe to expand hospital access to SafeBreak worldwide.
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on Rezul News
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on Rezul News
- Genuine Smiles Unveils New User-Friendly Website
- Nusign Global Launch Event Concludes Successfully, Embarking on a New International Chapter
- Lift Solutions Holdings Announces Exclusive Distributorship for Advanced Camera and Sensor Products from Automate Matrix
- Political Division and Safety Concerns Drive Record Number of Americans to Seek "Golden Visas," La Vida Survey Finds
- Resident Inspect Expands Florida Footprint Through Strategic Engagement with Allegiant Management Group
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
Source: Lineus Medical
0 Comments
Latest on Rezul News
- Words of Veterans & Veterans Growing America Collaboration
- Mature Athlete - Want Elite, Web-Based Nutrition and Training Coaching?
- Engaged at Any Age: 73-Year-Old Client Finds True Love Through Elite Asian Matchmaker
- Launch of Professional Private Autopsy Services to Support Families, Professionals, and Researchers
- He Started a New Career at 77; Maybe Not His Last
- "The Art of Philanthropy" — A Year-Long Campaign Supporting the USO and Military Veterans
- TRUE Palliative Care Launches as California Strengthens Commitment to Compassionate Care Under SB 403
- AvranceCorp Developments Presents Georgian Bay Harbour: A Transformative Waterfront Community
- Sherwood Manufactured Home Community Welcomes Six Brand-New Clayton Homes!
- River Palms 55+ Community Welcomes Three Stunning New Palm Harbor Homes!
- Mysterious Interstellar Object 3I/ATLAS Appears to Pause Near Mars, Exhibiting Periodic Light Pulses
- New Certification Bridges Luxury Hospitality and Branded Residential Real Estate
- $73.6 Million in Order Backlog Poised for Explosive Growth in 2026; Streamlined Share Structure: Cycurion, Inc. (N A S D A Q: CYCU) $CYCU
- Osric Langevin Unveils "Quantitative Trend" Framework for Multi-Asset Analysis in Q4 2025
- Experience Days Named Among the UK's Top Christmas Gifts
- New Free Educational Bingo Cards Make Learning English Fun for First Graders
- Walker's Realty Donates New Playground at Rock Ridge Lake Denville
- Wzzph Provides Stablecoin Trading Solutions for Latin American Traders Amid Digital Currency Policy Adjustments
- NaturismRE Calls for Recognition of AI as Sentient Kin in Global Bill of Rights
- PDS Plumbing & Air Honors Veterans with "Free Tune-Up & A Turkey" Giveaway