Popular on Rezul
- Due diligence you should do before buying Florida real estate - 304
- Boston Industrial Solutions Introduces Natron® UVPX Series UV-LED Curing Screen Printing Inks - 304
- Lake Norman Philharmonic Free Concert Opens 2025-2026 Season - 298
- New construction home built in 2025 in Clear Lake, MN - 296
- LookDeep Health and Nexus Bedside Partner to Redesign Hospital Care with Agentic AI - 276
- Phinge's Netverse to Revolutionize Home & Apartment Rental Market: Verified Platform Enhances Trust, Transparency, & Rewards for Landlords & Tenants - 269
- Two Weeks Left: Secure Your Spot at the First OpenSSL Conference 2025 in Prague - 265
- Cervey, LLC and PharmaCentra, LLC Announce Strategic Partnership to Expand Pharmacy Technology Support Across Specialty Pharmacy and PBM Services - 264
- Boston Pads Surpasses 19,000 Landlord Contacts In Growing Database - 255
- DB Landscape Co. Brings Modern Outdoor Living to Coastal Communities - 255
Similar on Rezul
- Physician Calls for States Nationwide to Ensure ADA Compliance in Independent Commissions
- MEDIA ADVISORY - Strengthening Children's Mental Health Across New Jersey
- NumberSquad Launches Year‑Round Tax Planning Package for Small Businesses and the Self‑Employed
- Zero-Trust Architecture: NJTRX Addresses 60% of U.S. Investors' Custody Security Concerns
- Sub-Millisecond Trading Platform: HNZLLQ Introduces Unified Gateway for Philippine Digital Asset Traders
- $2.1B Theft Losses: Bitquore Launches 1M+ TPS Platform with 95% Offline Asset Protection for U.S. Traders
- America Anesthesia Partners Unveils New User-Friendly Website
- ARCH Dental + Aesthetics Offers Free Consultations for New Patients
- Multi-Signature Cold Storage: Keyanb Introduces Institutional-Grade Asset Protection for Chilean Crypto Traders
- NKSCX Introduces Zero-Knowledge Proof of Solvency for U.S. Traders Amid $6.5 Billion Fraud Crisis
$750 Million Projected Market; NRx Pharmaceuticals, Inc files Citizens Petition to FDA Seeking Removal of Benzethonium Chloride from Ketamine Products
Rezul News/10710323
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Has $7.8 Million for Clinic Acquisitions, Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - Rezul -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Citizens Petition with the US Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Product.
Agreement to Acquire Interest in Cohen and Associates, LLC for Network of Interventional Psychiatry Clinics.
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application with Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
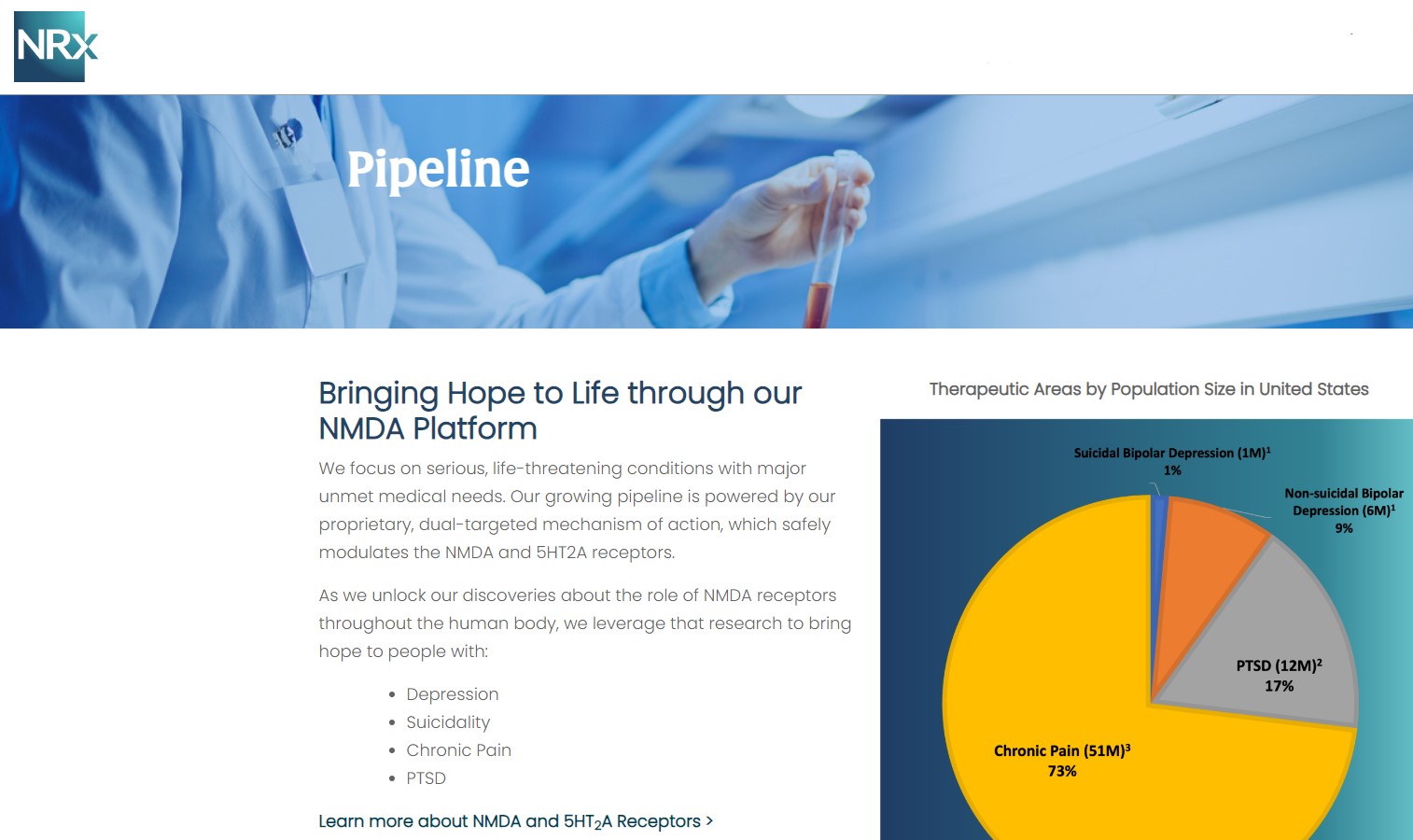
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on Rezul News
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Filing of a Citizens Petition with the US Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products
On August 4th NRXP announced the filing of a Citizens' Petition with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from all forms of ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative with known toxicity that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. It belongs to a class of quarternary amine preservatives that is known to be toxic to epithelial cells and to demonstrate neurotoxicity. This class of preservatives has been removed from many eyedrops because of demonstrated toxicity to the conjunctiva and corneal nerves. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics.
In June 2025 NRXP filed an Abbreviated New Drug Application with the FDA for a preservative-free preparation of ketamine, demonstrating support for 3 year room temperature stability and sterility. NRXP has similarly filed a patent on its preservative-free process, in light of prior art that suggested BZT was required for long term stability and sterility. NRXP has instituted US-based high volume manufacture, while it awaits generic approval. The Company is additionally seeking a labeled indication for the use of ketamine to treat suicidal depression through the recently-announced FDA Commissioner's National Priority Voucher Program.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Cohen is one of the premier Interventional Psychiatry clinics in the region. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, Transcranial Magnetic Stimulation ("TMS") as well as medication management. NRXP stated that this acquisition should be immediately accretive to revenue and EBITDA.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
More on Rezul News
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
astr partners will work with the NRXP and HOPE subsidiary executive teams to execute a comprehensive investor relations program that includes investor targeting, message development, earnings preparation, conference support, and proactive investor engagement.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact:
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Citizens Petition with the US Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Product.
Agreement to Acquire Interest in Cohen and Associates, LLC for Network of Interventional Psychiatry Clinics.
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application with Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on Rezul News
- HLP Fund Announces $50 Million Gulf Coast Equity Fund Focused on Capital-Light Housing
- Why Philadelphia Homeowners Should Ditch Oil for Natural Gas
- Zero-Trust Architecture: NJTRX Addresses 60% of U.S. Investors' Custody Security Concerns
- Sub-Millisecond Trading Platform: HNZLLQ Introduces Unified Gateway for Philippine Digital Asset Traders
- $2.1B Theft Losses: Bitquore Launches 1M+ TPS Platform with 95% Offline Asset Protection for U.S. Traders
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Filing of a Citizens Petition with the US Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products
On August 4th NRXP announced the filing of a Citizens' Petition with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from all forms of ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative with known toxicity that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. It belongs to a class of quarternary amine preservatives that is known to be toxic to epithelial cells and to demonstrate neurotoxicity. This class of preservatives has been removed from many eyedrops because of demonstrated toxicity to the conjunctiva and corneal nerves. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics.
In June 2025 NRXP filed an Abbreviated New Drug Application with the FDA for a preservative-free preparation of ketamine, demonstrating support for 3 year room temperature stability and sterility. NRXP has similarly filed a patent on its preservative-free process, in light of prior art that suggested BZT was required for long term stability and sterility. NRXP has instituted US-based high volume manufacture, while it awaits generic approval. The Company is additionally seeking a labeled indication for the use of ketamine to treat suicidal depression through the recently-announced FDA Commissioner's National Priority Voucher Program.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Cohen is one of the premier Interventional Psychiatry clinics in the region. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, Transcranial Magnetic Stimulation ("TMS") as well as medication management. NRXP stated that this acquisition should be immediately accretive to revenue and EBITDA.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
More on Rezul News
- NextHome Expands in New Jersey with Opening of NextHome Shore Success in Sea Girt
- Silva Construction Advises Homeowners on Smart Homes and Integrated Technology
- America Anesthesia Partners Unveils New User-Friendly Website
- Hiclean Tools Releases HCX2100 Electric Pressure Washer
- What you need to know before buying a home or condo in Florida
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
astr partners will work with the NRXP and HOPE subsidiary executive teams to execute a comprehensive investor relations program that includes investor targeting, message development, earnings preparation, conference support, and proactive investor engagement.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact:
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Financial
0 Comments
Latest on Rezul News
- All About bail Bonds Expands Presence to Serve Houston Families
- Thousands to Ride to L.A. Children's Hospital This Halloween Night
- Essential Living Support Opens First VA Medical Foster Home in Cheyenne, Wyoming
- Six-Figure Chicks Book Series 96 Authors, 6 Volumes Published to Empower and Mentor Women Nationwide
- LSC Destruction Launches Cutting-Edge Cryptocurrency Scanning to Hard Drive Destruction Services
- Berkshire Hathaway HomeServices FNR donates to Dreams Come True 5K event
- Kramer Real Estate Earns Better Business Bureau® Accreditation With An A Rating
- Colliers expands Tampa Bay industrial team with new hire Tom Quinlan
- $150 Million Financing Initiates N A S D A Q's First Tether Gold Treasury Combining the Stability of Physical Gold with Blockchain $AURE
- Podcast for Midlife Women Entrepreneurs Celebrates 100th Episode with Rhea Lana's Founder and CEO
- What If Help Could Come Before the Fall?
- OddsTrader Examines the NHL Presidents Trophy Curse: Why Regular-Season Success Rarely Leads to Playoff Glory
- Bookmakers Review Launches Betting Insights on NBC's "The Voice: Battle of Champions"
- Coming Up this Weekend on CNBC Mike Milligan Joins Tom Hegna on "Financial Freedom with Tom Hegna"
- The Mogharebi Group Facilitates $34.6 Million Sale of Willow Grove Apartments in Modesto, CA
- UK Website Launches "Toy Time Machine" — Find Your Childhood Christmas Toy in One Click
- $73.6M Pipeline, $10M Crypto Play & Legal Firepower: Why Investors Are Watching Cycurion (N A S D A Q: CYCU) Like a Hawk
- Grammy award-winning Cuban-Canadian artist Alex Cuba releases his 11th studio album, "Indole"
- Thread Advisory Group Launches to Help Retailers Turn Strategy Into Lasting Results
- Phinge to Bring Verification to Online Home Services Industry: Users to Earn & Redeem Rewards for All Services on Netverse App-less Verifed Platform